Duplication of a conserved mitochondrial enzyme gene arms parasitoid wasps with venom cytotoxicity and oogenesis regulation
Jiqiang Song, Zihan Qi, Chun He, Guilin Luo, Bo Yuan, Shan Xiao, Yi Yang, Fang Wang, Gongyin Ye , Qi Fang, and Zhichao Yan
PNAS, September 25, 2025; 122 (39) e2512820122; https://doi.org/10.1073/pnas.2512820122
Significance
Gene duplication drives evolutionary innovation through neofunctionalization, yet the underlying molecular mechanisms remain elusive. Here, we uncover how a conserved mitochondrial enzyme acquires venom cytotoxicity and oogenesis regulation through gene duplication in parasitoid wasps. The stepwise neofunctionalization process includes the loss of ancestral mitochondrial localization and enzyme activity, acquisition of a secretory signal peptide, shifts in expression patterns, positive selection, and the establishment of new protein–protein interactions. Notably, a neofunctionalized venom paralog exerts cytotoxicity against insect cells via interaction with insect chloride intracellular channels while showing no toxicity to human HEK293T cells, suggesting potential as a novel selective insecticidal gene. This study reveals how gene duplication enables functional diversification, providing a mechanistic framework for understanding evolutionary novelty in gene families.
Abstract
Gene duplication, followed by neofunctionalization, is a key mechanism driving the emergence of evolutionary novelties. Despite its significance, the molecular and functional processes underlying this phenomenon remain incompletely understood. By tracing the evolutionary history of cysteine-S-conjugate beta-lyase genes within the kynurenine aminotransferase family, we identified a gene duplication event in parasitoid wasps of the Chalcidoidea superfamily. Notably, a single-copy, highly conserved mitochondria-localized physiological gene underwent a significant duplication, resulting in one copy being recruited into the venom system and acquired cytotoxicity against wasps' hosts. Through this neofunctionalization process, we observed several key evolutionary changes, including loss of ancestral mitochondrial localization and enzyme activity, acquisition of a secretory signal peptide, shift in expression pattern, positive selection, and the establishment of evolutionary acquired protein–protein interactions. Additionally, we found that another duplicate copy was specialized in wasps' ovary and repurposed for oogenesis regulation. Our study offers a detailed insight into the genetic and molecular mechanisms that drive functional diversification during the evolution of gene families.
See https://www.pnas.org/doi/10.1073/pnas.2512820122
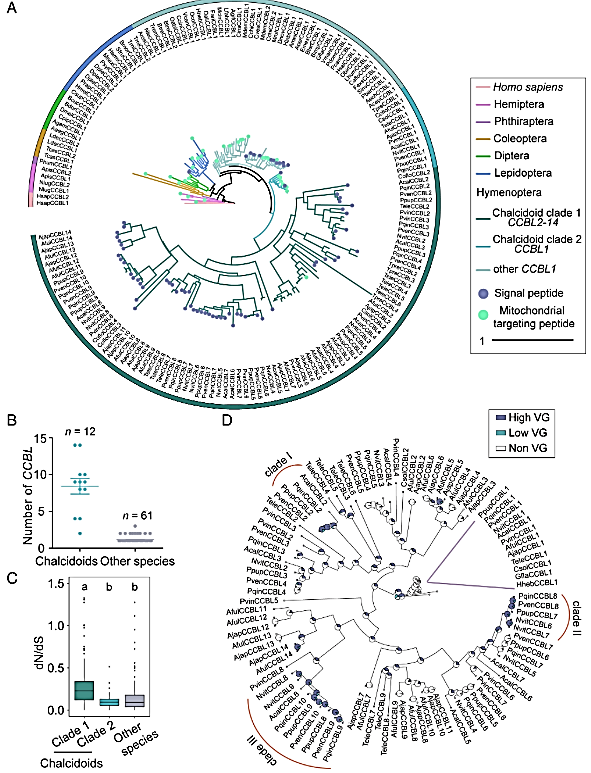
Figure 3: Accelerated evolution and expression alternation after CCBL duplication. (A) Phylogenetic tree of CCBL genes. Branches and labels with different colors represent different species, as indicated by the color code to the Right. Different shapes in different colors represent the signal peptide and mitochondrial targeting peptide of CCBLs. Selected species and corresponding abbreviations are listed in SI Appendix, Table S1. (B) Number of CCBL genes in chalcidoid parasitoid wasps and other species. The Mann–Whitney U test was used for comparison of CCBLs number: P < 0.001. (C) dN/dS ratios of clades between chalcidoids 1 and 2 as well as other species. (D) Ancestral state reconstruction of parasitoid wasps CCBL genes with ARD model based on the expression level of CCBLs in the venom glands (VG). Orange arcs indicate CCBLs recruited into the venom glands under Bayesian posterior probabilities (bpp).
Views: 35


